 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
| 製造番号 | 種類 | 製品説明 | 構造 | 純度 | 特徴 |
|---|---|---|---|---|---|
| COS-H52H3 | Human | Human Complement C1s Protein, His Tag | 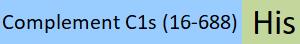 |

|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Conestat alfa | rhC1-INH | Approved | Pharming Group Nv | Rhucin, Esterasine, Ruconest | EU | Angioedemas, Hereditary | Pharming Group Nv | 2010-10-28 | Rejection of renal transplantation; Myocardial Infarction; Renal Insufficiency; Rejection of organ transplantation; Genetic Diseases, Inborn; Coronavirus Infections; Non-ST Elevated Myocardial Infarction; Angioedema; Angioedemas, Hereditary | Details |
| Sutimlimab | BIVV-009; TNT-009 | Approved | True North Therapeutics | ENJAYMO | United States | Anemia, Hemolytic, Autoimmune | Bioverativ Therapeutics Inc | 2022-02-04 | Hemolysis; Purpura, Thrombocytopenic, Idiopathic; Anemia, Hemolytic, Autoimmune; Pemphigoid, Bullous; Kidney Failure, Chronic | Details |
| Complement C1 inhibitor protein (Shire ViroPharma) | C1 INH-nf; C1-INH; SHP-616 | Approved | Sanquin Plasma Products Bv | Cinryze, Cetor | United States | Angioedemas, Hereditary | null | 2008-10-10 | Brain Injuries, Traumatic; Rejection of organ transplantation; Angioedemas, Hereditary | Details |
| C1 esterase inhibitor (Human, CSL Behring) | BI-3.012; CSL-830; CSL-842; CE-1145 | Approved | Csl Behring Llc | Berinert, Haegarda, Berinert 2000 | Japan | Angioedemas, Hereditary | Csl Behring Llc | 1990-06-29 | Hereditary Angioedema Types I and II; Angioedemas, Hereditary | Details |
| Conestat alfa | rhC1-INH | Approved | Pharming Group Nv | Rhucin, Esterasine, Ruconest | EU | Angioedemas, Hereditary | Pharming Group Nv | 2010-10-28 | Rejection of renal transplantation; Myocardial Infarction; Renal Insufficiency; Rejection of organ transplantation; Genetic Diseases, Inborn; Coronavirus Infections; Non-ST Elevated Myocardial Infarction; Angioedema; Angioedemas, Hereditary | Details |
| Sutimlimab | BIVV-009; TNT-009 | Approved | True North Therapeutics | ENJAYMO | United States | Anemia, Hemolytic, Autoimmune | Bioverativ Therapeutics Inc | 2022-02-04 | Hemolysis; Purpura, Thrombocytopenic, Idiopathic; Anemia, Hemolytic, Autoimmune; Pemphigoid, Bullous; Kidney Failure, Chronic | Details |
| Complement C1 inhibitor protein (Shire ViroPharma) | C1 INH-nf; C1-INH; SHP-616 | Approved | Sanquin Plasma Products Bv | Cinryze, Cetor | United States | Angioedemas, Hereditary | null | 2008-10-10 | Brain Injuries, Traumatic; Rejection of organ transplantation; Angioedemas, Hereditary | Details |
| C1 esterase inhibitor (Human, CSL Behring) | BI-3.012; CSL-830; CSL-842; CE-1145 | Approved | Csl Behring Llc | Berinert, Haegarda, Berinert 2000 | Japan | Angioedemas, Hereditary | Csl Behring Llc | 1990-06-29 | Hereditary Angioedema Types I and II; Angioedemas, Hereditary | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Riliprubart | SAR-445088; TNT-020; BIVV-020 | Phase 3 Clinical | True North Therapeutics | Purpura, Thrombocytopenic, Idiopathic; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Polyneuropathies; Rejection of organ transplantation; Anemia, Hemolytic | Details |
| C1 esterase inhibitor (Boya Bio-Pharmaceutical) | Phase 3 Clinical | Boya Bio-Pharmaceutical Group Co Ltd | Angioedemas, Hereditary | Details | |
| Complement-C1 inhibitor protein (Octapharma) | Phase 2 Clinical | Octapharma | Angioedemas, Hereditary | Details | |
| DNTH-103 | DNTH-103; DNTH103 | Phase 2 Clinical | Dianthus Therapeutics Inc | Myasthenia Gravis; Autoimmune Diseases | Details |
| C1 esterase inhibitor (Chengdu Institute of Biological Products) | Phase 1 Clinical | Chengdu Institute Of Biological Products Co Ltd | Angioedemas, Hereditary | Details | |
| Riliprubart | SAR-445088; TNT-020; BIVV-020 | Phase 3 Clinical | True North Therapeutics | Purpura, Thrombocytopenic, Idiopathic; Polyradiculoneuropathy, Chronic Inflammatory Demyelinating; Polyneuropathies; Rejection of organ transplantation; Anemia, Hemolytic | Details |
| C1 esterase inhibitor (Boya Bio-Pharmaceutical) | Phase 3 Clinical | Boya Bio-Pharmaceutical Group Co Ltd | Angioedemas, Hereditary | Details | |
| Complement-C1 inhibitor protein (Octapharma) | Phase 2 Clinical | Octapharma | Angioedemas, Hereditary | Details | |
| DNTH-103 | DNTH-103; DNTH103 | Phase 2 Clinical | Dianthus Therapeutics Inc | Myasthenia Gravis; Autoimmune Diseases | Details |
| C1 esterase inhibitor (Chengdu Institute of Biological Products) | Phase 1 Clinical | Chengdu Institute Of Biological Products Co Ltd | Angioedemas, Hereditary | Details |
This web search service is supported by Google Inc.